© Alexandra Chambers | Neurotopia CIC | January 2026
In connective tissue divergence, immune sensitivity, or genomically divergent bodies, fibrosis is one of the most damaging long-term outcomes. Here I will explain what actually drives that scarring process deep in the tissues.
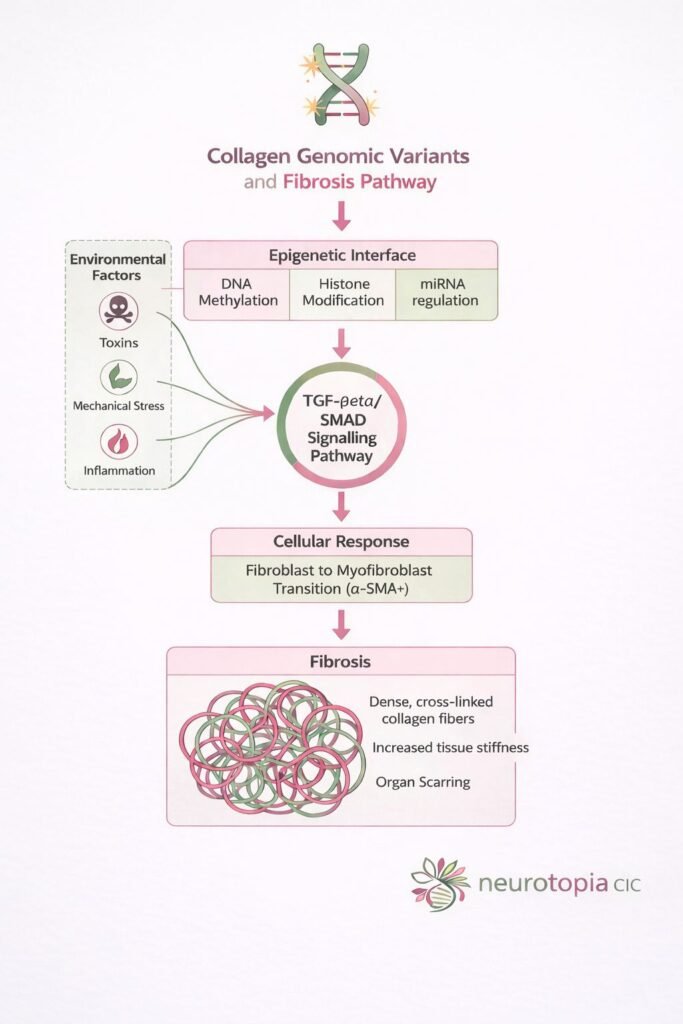
At the heart of the fibrosis response is a powerful molecular communication route called the TGF-β / SMAD signalling pathway – a system that tells your cells when to switch on collagen production, when to remodel tissue, and when to scar.
This article walks you through what the SMAD pathway is, how it works, and why it’s so critical for people with genomic sensitivities like COL1A1, COL3A1, or TGFBR2 variants – as well as those exposed to environmental stressors.
![]() The Basics: What is the SMAD Pathway?
The Basics: What is the SMAD Pathway?
SMAD proteins are messengers. They carry a signal from the surface of a cell – after it receives a warning (such as inflammation or damage) – straight into the nucleus, where your genes are read and expressed.
However, SMAD doesn’t act alone. It’s part of the TGF-β (Transforming Growth Factor-beta) pathway – the master switch in most fibrotic processes.
![]() Step-by-Step: How the SMAD Pathway Triggers Fibrosis
Step-by-Step: How the SMAD Pathway Triggers Fibrosis
-TGF-β is released
When the body experiences inflammation, mechanical stress, or toxic exposure, immune cells and tissues begin releasing TGF-β.
-Cell receptors receive the signal
TGF-β binds to specific surface receptors (TGF-βRI and TGF-βRII), activating them.
SMAD2/3 become phosphorylated.
These are “receptor-SMADs” (R-SMADs). Once activated, they’re tagged to carry the signal forward.
SMAD complex forms with SMAD4
The active SMAD2/3 pair with SMAD4 – the co-SMAD – and travel into the nucleus.
-Gene expression changes
Inside the nucleus, the SMAD complex activates genes responsible for:
Collagen production (COL1A1, COL3A1).
Myofibroblast conversion (α-SMA).
Matrix cross-linking enzymes (e.g. LOX)
This leads to tissue stiffening, scarring, and eventually, organ-level dysfunction if left unchecked.
![]() Why is This Pathway Crucial for Divergent Populations?
Why is This Pathway Crucial for Divergent Populations?
The SMAD pathway intersects with both genomic and epigenetic systems. That means:
People with collagen gene variants (e.g. COL1A1, COL3A1) are already primed for a stronger fibrotic response.
Environmental inputs like toxins, pharma overload, trauma or mechanical strain can act as triggers.
Epigenetic mechanisms – like DNA methylation or miRNA (e.g. miR-21) – modulate how sensitive the pathway is.
This creates a perfect storm: a body genomically wired to respond more strongly to stress, paired with epigenetic switches that have been flipped by chronic exposure.
![]() SMAD Pathway in EDS, Loeys-Dietz, and More
SMAD Pathway in EDS, Loeys-Dietz, and More
Loeys-Dietz syndrome, vascular EDS, and generalised connective tissue fragility often involve:
Differences in TGF-β signalling.
Loss of control in the SMAD cascade.
Misrepair and fibrosis following micro-injury.
This explains why some bodies scar more, heal poorly, or develop stiffened tissues even without visible trauma. It’s not random – it’s written in the signalling.
If your terrain is genomically divergent – it is often more permeable and susceptible to pharmaceuticals, toxins, bacteria or systemic inflammation – the SMAD pathway can act like a locked loop, reinforcing damage instead of resolving it.
This is why early intervention, antioxidant buffering, and epigenetic support matter. It’s not just about blocking TGF-β. It’s about restoring balance to the signalling system – and the terrain it responds to.
![]() Neurotopia CIC | 2026
Neurotopia CIC | 2026
#neurodiversity#neurodivergent#Neurotopia#science#genomics#adhd#autism#epigenetics