© Alexandra Chambers | Neurotopia CIC | December 2025
Disclaimer:
Every individual’s biology is unique. Genetic variation, methylation status, nutrient processing, and environmental factors all influence what supports or disrupts internal systems. What strengthens one person’s blood–brain barrier may overwhelm another’s. This article is written from a divergent genomics perspective, with emphasis on terrains that are often under-recognised in conventional frameworks. Always adapt support strategies to your own body’s feedback, genomic insights, and lived experience.
The blood–brain barrier (BBB) is often treated in textbooks as a static anatomical feature-an impermeable wall protecting the brain from toxins, pathogens, and immune chaos. In reality, it is a living, dynamic interface: selectively permeable, metabolically active, and governed by a delicate balance of structural proteins, endothelial tight junctions, vascular tone, and immune signalling.
For people with connective tissue divergence (collagen variants)-whether formally diagnosed (e.g. Ehlers–Danlos syndromes) or subclinical-the integrity of this barrier is often more fragile. In an industrial age marked by chemical load, synthetic exposures, and cumulative epigenetic insults, that vulnerability is increasingly stressed.
Connective Tissue, Collagen, and the BBB
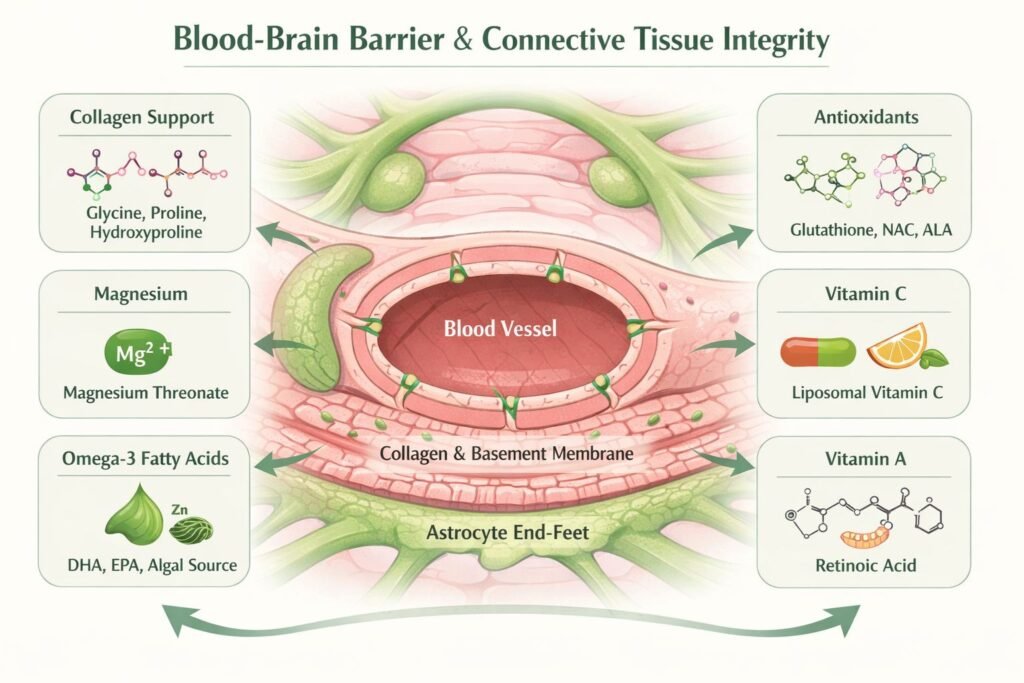
At its core, the BBB consists of endothelial cells lining cerebral blood vessels, supported by a basement membrane (basal lamina), pericytes, and astrocytic end-feet. Structural coherence depends heavily on collagen- particularly type IV within the basement membrane-alongside elastin, laminins, and glycoproteins of the extracellular matrix.
Subtle genetic variants affecting collagen synthesis, hydroxylation, or cross-linking (e.g. COL4A1/2, PLOD1, FKBP14) may weaken this matrix, increasing permeability and dysregulating molecular traffic between blood and brain.
In neurodivergent populations, connective tissue divergence often co-exists silently. A person may not meet hypermobility criteria yet still exhibit molecular-level fragility. When this fragility involves the BBB, susceptibility to environmental insults increases-mould toxins, heavy metals, synthetic folic acid, or persistent viral antigens may bypass weakened defences and provoke chronic neuroimmune activation.
Key Supports for BBB Resilience ![]()
Rather than seeking a single “cure,” the focus is terrain support: restoring the metabolic and structural conditions that allow the barrier to function as intended.
Evidence supports a multifactorial model:
1. Amino Acids for Collagen Synthesis
Glycine, proline, and hydroxyproline are foundational to collagen. Targeted support (e.g. glycine powders or collagen peptides) may benefit individuals with connective tissue fragility-not only for skin and joints, but for vascular linings and basement membranes, including the BBB.
2. Magnesium
Magnesium regulates endothelial tone, mitochondrial function, and inflammatory signalling. Deficiency destabilises tight junctions and increases oxidative stress. Magnesium threonate may offer particular neurological value due to its own ability to penetrate the BBB.
3. Omega-3 Fatty Acids (DHA, EPA) ![]()
DHA is a critical component of neuronal and endothelial membranes. Omega-3s reduce endothelial activation, modulate inflammation, and improve junctional protein expression. Those with fatty-acid metabolism divergence or sensory aversion may tolerate algal sources better than fish oils.
4. Zinc
Zinc supports tight junction integrity, matrix metalloproteinase regulation, and anti-inflammatory signalling. Deficiency is linked to both “leaky gut” and “leaky brain.” Zinc also counterbalances copper-driven oxidative stress, relevant in ceruloplasmin or iron-handling divergence.
5. Antioxidant Support (Glutathione, NAC, Alpha-Lipoic Acid)
Chronic oxidative stress weakens barrier structures. Glutathione support-directly or via precursors like NAC-helps neutralise ROS and reduce immune-driven permeability shifts.
6. Liposomal Vitamin C ![]()
Vitamin C is essential for proline and lysine hydroxylation during collagen synthesis-critical for microvascular and connective tissue stability. Liposomal forms improve intracellular delivery with reduced gastrointestinal burden.
7. Vitamin A (Retinoic Acid) ![]()
Retinoic acid influences tight-junction gene expression and immune gating. Deficiency or imbalance can disrupt barrier signalling. Caution is essential in those with liver compromise or methylation pathway vulnerability.
8. Calcium and Vascular Tone ![]()
Calcium supports junctional adhesion and endothelial signalling, but balance is key. Inflammatory states may dysregulate calcium handling via voltage-gated calcium channels (VGCCs)-particularly relevant in channelopathies.
9. The Gut–Brain Axis ![]()
Gut barrier disruption often precedes BBB compromise. Dysbiosis, histamine overload, or chronic gut inflammation elevate systemic immune tone and destabilise cerebral barriers.
Support may include L-glutamine, histamine-aware nutrition, glycogen-supportive fibres (e.g. green banana flour), and mould-toxin mitigation.
10. Stress Load and Cortisol ![]()
Chronic cortisol exposure loosens tight junctions and amplifies inflammatory signalling. Nervous-system regulation-sleep, breathwork, trauma-informed approaches, and carefully selected adaptogens-matters as much as nutrients.
Hidden Saboteurs: What to Avoid ![]()
Synthetic folic acid (PGA / UMFA / ‘vitamin’ B9) from fortified foods may disrupt endothelial regulation, particularly in people with MTHFR variants or unmetabolised folic acid accumulation.
Fluoride, often added to water and dental products under the banner of tooth protection, is increasingly recognised for its disruptive effects on connective tissue, mineral balance, and neurodevelopment – especially in genomically sensitive individuals. It binds to calcium and interferes with collagen cross-linking, subtly weakening the structural matrix of tissues like bone, fascia, and dentin. In those with connective tissue variants, this may compound fragility or sensory overload. Fluoride also accumulates in the pineal gland, contributing to early calcification and potential disruption of melatonin regulation, sleep, and light sensitivity. While dose and susceptibility vary, its overlooked role in tissue integrity and terrain fragility deserves renewed scrutiny.
Mould toxins (e.g. ochratoxin A) readily penetrate compromised barriers and activate microglia.
Persistent viral antigens (e.g. EBV, spike protein fragments) may circulate longer in divergent terrains, sustaining neuroinflammation.
Beyond Pathology
The question is not how to “fix” connective tissue divergence -but how to support different bodies to function optimally in an overstimulated, chemically burdened world.
Heightened sensitivity often reflects reduced buffering capacity, not dysfunction. With appropriate terrain support, these systems can be remarkably adaptive, perceptive, and responsive.
Supporting the blood brain barrier is about restoring coherence, integrity, and respect for biological diversity.
Divergent Genomics by Neurotopia ![]() https://www.facebook.com/groups/1305414697616716/?ref=share
https://www.facebook.com/groups/1305414697616716/?ref=share
#neurodiversity#neurodivergent#BBB#connectivetissue#bloodbrainbarrier#autism#adhd#orthomolecularmedicine#divergentgenomicsbyneurotopia#divergent#science#terrain#metabolism#neuroimmunology